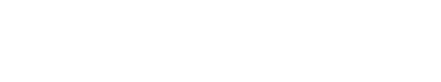White carbonate veins where carbon dioxide has been transformed into rock show in a dark peridotite deposit in Oman. This process of carbonation produces an increase in the volume of solid rock and offers a way to capture and sequester carbon dioxide. The reaction causes fractures in the remaining peridotite and the breaks provide more access to reactive surfaces
by Beth Ulion
Feb 03, 2009
Billions of tons of carbon dioxide could be captured by rocks in a natural chemical reaction that humans can speed up, say researchers from Columbia University.
Peridotite, a rock found in the Earth’s mantle, is exposed at the surface in many places on the planet. In the Middle East sultanate of Oman, this mineral is naturally converting an estimated 100,000 tons of the greenhouse gas carbon dioxide into rock each year. That’s enough to soak up the CO2 emissions from burning more than 10 million gallons of gasoline.

White carbonate veins where carbon dioxide has been transformed into rock show in a dark peridotite deposit in Oman. This process of carbonation produces an increase in the volume of solid rock and offers a way to capture and sequester carbon dioxide. The reaction causes fractures in the remaining peridotite and the breaks provide more access to reactive surfaces
The potential for peridotite is reported in a recent study, “In situ carbonation of peridotite for CO2 storage,” published in the Proceedings of the National Academy of Sciences. The Columbia researchers measured the rate of natural carbon capture in the rocks found in Oman and also assessed the prospects of increasing the rate of reaction.
Drilling, the artificial input of the greenhouse gas and an increase of temperature in the rock can accelerate the process and create a “significant sink for atmospheric CO2,” stated researchers Peter Kelemen and Jurg Matter in the published study.
Lead researcher Peter Kelemen, a professor of earth and environmental sciences at the Lamont-Doherty Earth Observatory of Columbia University, discusses the future of this technology.
What are the next steps for putting the peridotite discovery to use?
We need to do a pilot experiment in natural peridotite over smaller scales of at least tens to hundreds of meters before expanding to volumes of cubic kilometers that could be possible. This is needed to evaluate whether the reaction can reach a “self-cracking” regime in which the volume change due to carbonation creates new pore space and exposes fresh minerals for reaction.
Your study says that the peridotite in the Sultanate of Oman alone has the ability to absorb 4 billion tons of CO2. Could you put that into perspective?

Peter Kelemen doing field work on carbon capture in the peridotite deposits of northern California.
Human output to the atmosphere is circa 30 billion tons of CO2 per year at present. This is increasing every year.
Where are peridotite deposits worldwide?
Peridotite is abundant on all continents, except maybe Antarctica. There are plenty of places with enough cubic kilometers of peridotite to potentially have a huge impact on the CO2 budget of the Earth. In the U.S., peridotite in significant quantities is mostly in California, Oregon, Washington, and maybe Montana.
What is the CO2 absorption potential worldwide?
Per cubic kilometer of rock per year, it is an absurdly large number. If you could carbonate every molecule of magnesium in the peridotite in Oman alone it would be able to take up the amount of CO2 currently released in a year by humans, every year for one-thousand years. Limits on achieving this potential have to due with practical concerns such as cost.
Could you lay out a possible scenario for the implementation of this technology?

Peridotite is found at the top of the mantle layer and breaks through the crust in some areas of the world.
Imagine you were in Oman. First, the pilot study would need to be successful. Second, maybe oil companies in the country would want to negate their carbon footprint. They would invest in a project like this. There would be negligible transport costs since it is in the country. Third, a societal call for carbon sequestration creates an effective carbon trading market which increases investment in these projects and covers costs of CO2 transport from other areas, including shipping and pipelines.
How quickly could development of this technology make a difference in curbing CO2 emissions?
Let’s do the pilot experiment, which has a 2 to 4-year time horizon and then go from there. Beyond the pilot experiment, further development becomes a societal issue. If the pilot is successful, practical implementation could take place within a few years from an engineering standpoint. But it would almost certainly require an international carbon credit system, legal work on ownership and liability and extensive review of potential sites for environmental and social impacts.
No one is going to do this for free on the scale needed for global impact, which is why the carbon credit trade is so essential.
When people hear “CO2 rock sponge” they picture everyone having a piece of this rock on their front porch just sucking up CO2. Could you explain why this isn’t feasible?
At natural geological rates, the peridotite in Oman, which is the largest deposit on the surface of the Earth, captures 100,000 tons of CO2 per year naturally. To get it to go faster requires elevated temperature and pressure. These are available deep underground but not on the surface.
The reaction between the rock and CO2 creates heat, which can be used to maintain optimal, high temperatures at depth.
Could artificial peridotite be manufactured?
Not at any reasonable cost. Why do that when it is available for free on the surface of the Earth?
Is this a system that would be used for sequestration of CO2 directly produced by humans from a power plant or would it be able to pull CO2 from the atmosphere?
We are looking at the idea of using shallow seawater, rather than CO2 transported from a power plant. Using seawater would be very inefficient, in terms of tons of CO2 per cubic kilometer of rock per year, but it would avoid the cost of CO2 capture technology in power plants, and the cost of transporting CO2.
Shallow seawater is in CO2 exchange equilibrium with the atmosphere and the air moves CO2 around the globe for free. As a result, it might be cheaper to simply involve more cubic kilometers of rock by drilling a lot more holes. We need to look at the cost balance between these two options: purified CO2 vs seawater.
Are there any concerns about the impact of this process on surrounding ecosystems?
Yes, the process we propose would probably disrupt local water supplies. This is especially true if pumping of seawater is involved. The host country would have to look at that balance. We are not aware of any toxic byproducts – solid carbonates are inert, stable and safe. The water that goes through the natural system that we are studying is not harmful and, after its reaction with the atmosphere, has a lower pH. It is used for irrigation by the Omanis.
Volume changes in the rock due to sub-surface reaction might also deform the surface. Conducting the entire process just offshore would mitigate a lot of these potential impacts.